前瞻科技 ›› 2024, Vol. 3 ›› Issue (3): 97-110.DOI: 10.3981/j.issn.2097-0781.2024.03.008
程醒,*( ), 李曈,*(
), 李曈,*( ), 司知蠢, 干林, 吕伟, 康飞宇†(
), 司知蠢, 干林, 吕伟, 康飞宇†( )
)
收稿日期:2024-06-24
修回日期:2024-07-01
出版日期:2024-09-20
发布日期:2024-09-18
通讯作者:
†
作者简介:程醒,博士。主要从事高镍正极材料结构改性及匹配聚合物固态电解质等研究。电子信箱:chengxing@sz.tsinghua.edu.cn。基金资助:
CHENG Xing,*( ), LI Tong,*(
), LI Tong,*( ), SI Zhichun, GAN Lin, LÜ Wei, KANG Feiyu†(
), SI Zhichun, GAN Lin, LÜ Wei, KANG Feiyu†( )
)
Received:2024-06-24
Revised:2024-07-01
Online:2024-09-20
Published:2024-09-18
Contact:
†
About author:* Equivalent contribution author
摘要:
碳材料作为电极材料或关键组分在诸多能源存储与转化器件中发挥着不可或缺的作用。然而,传统碳材料存在的结构单一、富含缺陷和织构无序等问题严重制约了相关器件性能的提升,难以满足新能源和电动汽车产业的快速发展需求。针对上述问题,文章提出了微纳超结构碳的概念和设计思想,采用结构纳米化、复合化、有序化设计和功能导向组装,构建碳材料跨越“纳-微-宏”的多层次孔道、多尺度网络、多组分界面,获得具有“精准定制、层次有序、厚密联通、多相耦合”基本特征的微纳超结构碳。同时,文章全面综述了微纳超结构碳材料在能源存储与转换器件中应用的国内外最新研究进展,涵盖了锂/钠离子电池、超级电容器、固态电池、水系电池以及氢能转换技术等关键领域,并对未来储能用碳材料的发展方向和应用模式作出展望。
程醒, 李曈, 司知蠢, 干林, 吕伟, 康飞宇. 能源存储与转化用微纳超结构碳:现状与建议[J]. 前瞻科技, 2024, 3(3): 97-110.
CHENG Xing, LI Tong, SI Zhichun, GAN Lin, LÜ Wei, KANG Feiyu. Status and Prospect on Micro-nano Superstructured Carbon for Energy Storage and Conversion[J]. Science and Technology Foresight, 2024, 3(3): 97-110.
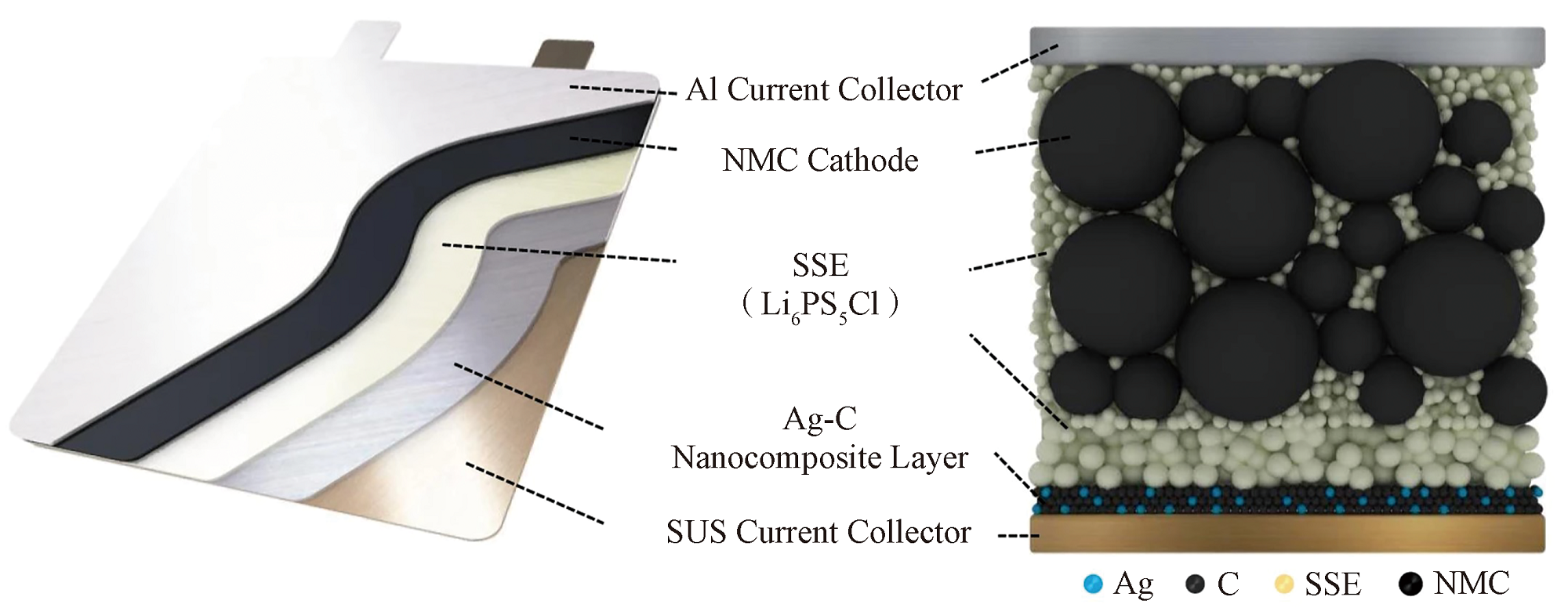
图3 包含高电容(>6.8 mA·h/cm2)的LiNi0.9Co0.05Mn0.05O2 (NMC)正极、硫化物固态电解质和不需要过量锂的Ag-C纳米复合负极层组成
Fig. 3 Structure composed of high-capacitance (>6.8 mA·h/cm2) LiNi0.9Co0.05Mn0.05O2 (NMC) cathode electrode, sulfide solid electrolyte, and Ag-C nanocomposite anode electrode requiring no excessive lithium

图4 以稳定的锌负极为目标的三维分层结构石墨烯矩阵设计示意图 Zn沉积在裸Zn(a)、纵向三维石墨烯矩阵(b)和径向三维石墨烯矩阵(c)上。
Fig. 4 Three-dimensional layered graphene matrix for stabling zinc anode electrode

图5 面向燃料电池低Pt催化剂的微纳米碳载体材料设计 图(a)表示不同碳载体(Vulcan与HSC碳)中Pt催化剂空间分布的STEM电子三维重构( 表示处于孔道内部的Pt, 表示暴露于载体表面的Pt);图(b)表示通过设计具有合适孔道深度的新型介孔碳载体优化Pt催化剂( , 分别表示较高活性和较低活性)与Nafion离子聚合物( )的界面结构。
Fig. 5 Design of micro-nano carbon material for low Pt catalysts in fuel cells

图6 面向燃料电池非贵金属催化剂的微纳米碳载体材料设计 图(a)和图(b)表示采用MOF为前驱体结合模板法制备的Fe-N-C单原子催化剂及其在燃料电池中的电子/质子/物质输运过程示意图;图(c)表示碳载体表面负载Fe单原子催化剂的高分辨STEM图;图(d)表示调制Fe单原子与碳载体之间的配位结构实现高稳定性的非贵金属催化剂。
Fig. 6 Design of micro-nano carbon material for non-precious metal catalysts in fuel cells

图7 面向电解水制氢的“亲水/疏气”多孔碳基电极材料设计 图(a)表示传统电极催化表面,气泡的黏附导致催化活性位点被覆盖;图(b)表示通过微纳米尺度的超亲水/疏气多孔碳基电极设计,有效减小气泡的黏附,显著提升析氢反应的催化性能。
Fig. 7 Design of “hydrophilic/hydrophobic” porous carbon electrode for hydrogen production by water electrolysis
| [1] | Kong D B, Lü W, Liu R L, et al. Superstructured carbon materials: Design and energy applications[J]. Energy Materials and Devices, 2023, 1(2), doi: 10.26599/emd.2023.9370017. |
| [2] | Zhao L, Ding B C, Qin X Y, et al. Revisiting the roles of natural graphite in ongoing lithium-ion batteries[J]. Advanced Materials, 2022, 34(18), doi: 10.1002/adma.202106704. |
| [3] | Cheng Y W, Lin C K, Chu Y C, et al. Electrically conductive ultrananocrystalline diamond-coated natural graphite-copper anode for new long life lithium-ion battery[J]. Advanced Materials, 2014, 26(22): 3724-3729. |
| [4] | Yoshio M, Wang H Y, Fukuda K. Spherical carbon-coated natural graphite as a lithium-ion battery-anode material[J]. Angewandte Chemie (International Ed in English), 2003, 42(35): 4203-4206. |
| [5] | Cai W L, Yan C, Yao Y X, et al. Rapid lithium diffusion in Order@Disorder pathways for fast-charging graphite anodes[J]. Small Structures, 2020, 1(1), doi: 10.1002/sstr.202070001. |
| [6] | Jia H P, Li X L, Song J H, et al. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes[J]. Nature Communications, 2020, 11, doi: 10.1038/s41467-020-15217-9. |
| [7] |
Liu N, Wu H, Mcdowell M T, et al. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes[J]. Nano Letters, 2012, 12(6): 3315-3321.
DOI PMID |
| [8] |
Liu N, Lu Z D, Zhao J, et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes[J]. Nature Nanotechnology, 2014, 9: 187-192.
DOI PMID |
| [9] | Xu D W, Chu X D, He Y B, et al. Enhanced performance of interconnected LiFePO4/C microspheres with excellent multiple conductive network and subtle mesoporous structure[J]. Electrochimica Acta, 2015, 152: 398-407. |
| [10] | Park S, Oh J, Kim J M, et al. Facile preparation of cellulose nanofiber derived carbon and reduced graphene oxide co-supported LiFePO4 nanocomposite as enhanced cathode material for lithium-ion battery[J]. Electrochimica Acta, 2020, 354, doi: 10.1016/j.electacta.2020.136707. |
| [11] | Lalia B S, Shah T, Hashaikeh R. Microbundles of carbon nanostructures as binder free highly conductive matrix for LiFePO4 battery cathode[J]. Journal of Power Sources, 2015, 278: 314-319. |
| [12] | Komaba S, Murata W, Ishikawa T, et al. Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries[J]. Advanced Functional Materials, 2011, 21(20): 3859-3867. |
| [13] | Xu T Y, Qiu X, Zhang X, et al. Regulation of surface oxygen functional groups and pore structure of bamboo-derived hard carbon for enhanced sodium storage performance[J]. Chemical Engineering Journal, 2023, 452, doi: 10.1016/j.cej.2022.139514. |
| [14] | Zhang S W, Lü W, Luo C, et al. Commercial carbon molecular sieves as a high performance anode for sodium-ion batteries[J]. Energy Storage Materials, 2016, 3: 18-23. |
| [15] | Li Q, Liu X, Tao Y, et al. Sieving carbons promise practical anodes with extensible low-potential plateaus for sodium batteries[J]. National Science Review, 2022, 9(8), doi: 10.1093/nsr/nwac084. |
| [16] | Shang L, Yuan R L, Liu H Y, et al. Precursor screening of fruit shell derived hard carbons for low-potential sodium storage: A low lignin content supports the formation of closed pores[J]. Carbon, 2024, 223, doi: 10.1016/j.carbon.2024.119038. |
| [17] | Yamamoto H, Muratsubaki S, Kubota K, et al. Synthesizing higher-capacity hard-carbons from cellulose for Na- and K-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(35): 16844-16848. |
| [18] |
Alvin S, Yoon D, Chandra C, et al. Revealing sodium ion storage mechanism in hard carbon[J]. Carbon, 2019, 145: 67-81.
DOI |
| [19] | Zhang B, Ghimbeu C M, Laberty C, et al. Correlation between microstructure and Na storage behavior in hard carbon[J]. Advanced Energy Materials, 2016, 6(1), doi: 10.1002/aenm.201501588. |
| [20] | Xu F, Tang Z W, Huang S Q, et al. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage[J]. Nature Communications, 2015, 6, doi: 10.1038/ncomms8221. |
| [21] | Lei Y, Huang Z H, Yang Y, et al. Porous mesocarbon microbeads with graphitic shells: Constructing a high-rate, high-capacity cathode for hybrid supercapacitor[J]. Scientific Reports, 2013, 3, doi: 10.1038/srep02477. |
| [22] | Ye L, Liang Q H, Lei Y, et al. A high performance Li-ion capacitor constructed with Li4Ti5O12/C hybrid and porous graphene macroform[J]. Journal of Power Sources, 2015, 282: 174-178. |
| [23] | 任晓龙. 锂离子电容器钒基氧化物碳复合电极材料的研究[D]. 北京: 清华大学, 2020. |
| Ren X L. Research on vanadium-based oxide-carbon composite electrode materials for lithium-ion capacitors[D]. Beijing: Tsinghua University, 2020. (in Chinese) | |
| [24] | Zhan C Z, Liu W, Hu M X, et al. High-performance sodium-ion hybrid capacitors based on an interlayer-expanded MoS2/rGO composite: Surpassing the performance of lithium-ion capacitors in a uniform system[J]. NPG Asia Materials, 2018, 10: 775-787. |
| [25] | Chen L H, Zhang J, Tong R A, et al. Excellent Li/garnet interface wettability achieved by porous hard carbon layer for solid state Li metal battery[J]. Small, 2022, 18(8), doi: 10.1002/smll.202106142. |
| [26] | Lee Y G, Fujiki S, Jung C, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes[J]. Nature Energy, 2020, 5: 299-308. |
| [27] | Liu Y, An X F, Yang K, et al. Achieving a high loading of cathode in PVDF-based solid-state battery[J]. Energy & Environmental Science, 2024, 17(1): 344-353. |
| [28] | Gomari S, Esfandeh M, Ghasemi I. All-solid-state flexible nanocomposite polymer electrolytes based on poly (ethylene oxide): Lithium perchlorate using functionalized graphene[J]. Solid State Ionics, 2017, 303: 37-46. |
| [29] | Zhai P B, Yang Z L, Wei Y, et al. Two-dimensional fluorinated graphene reinforced solid polymer electrolytes for high-performance solid-state lithium batteries[J]. Advanced Energy Materials, 2022, 12(42), doi: 10.1002/aenm.202200967. |
| [30] | Xu C J, Li B H, Du H D, et al. Energetic zinc ion chemistry: The rechargeable zinc ion battery[J]. Angewandte Chemie (International Ed in English), 2012, 51(4): 933-935. |
| [31] | Lee J H, Kim R, Kim S, et al. Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries[J]. Energy & Environmental Science, 2020, 13(9): 2839-2848. |
| [32] | Mu Y B, Li Z, Wu B K, et al. 3D hierarchical graphene matrices enable stable Zn anodes for aqueous Zn batteries[J]. Nature Communications, 2023, 14, doi: 10.1038/s41467-023-39947-8. |
| [33] | Zhang H, Luo Z, Deng W T, et al. Interfacial reconstruction via electronegative sulfonated carbon dots in hybrid electrolyte for ultra-durable zinc battery[J]. Chemical Engineering Journal, 2023, 461, doi: 10.1016/j.cej.2023.142105. |
| [34] | Nian Q S, Sun T J, Li Y C, et al. Regulating frozen electrolyte structure with colloidal dispersion for low temperature aqueous batteries[J]. Angewandte Chemie (International Ed in English), 2023, 62(9), doi: 10.1002/anie.202217671. |
| [35] | Padgett E, Andrejevic N, Liu Z Y, et al. Editors’ choice—Connecting fuel cell catalyst nanostructure and accessibility using quantitative cryo-STEM tomography[J]. Journal of the Electrochemical Society, 2018, 165(3): F173-F180. |
| [36] |
Girod R, Lazaridis T, Gasteiger H A, et al. Three-dimensional nanoimaging of fuel cell catalyst layers[J]. Nature Catalysis, 2023, 6: 383-391.
DOI PMID |
| [37] | Yarlagadda V, Carpenter M K, Moylan T E, et al. Boosting fuel cell performance with accessible carbon mesopores[J]. ACS Energy Letters, 2018, 3(3): 618-621. |
| [38] |
Ott S, Orfanidi A, Schmies H, et al. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells[J]. Nature Materials, 2020, 19: 77-85.
DOI PMID |
| [39] |
Jiao L, Li J K, Richard L L, et al. Chemical vapour deposition of Fe-N-C oxygen reduction catalysts with full utilization of dense Fe-N4 sites[J]. Nature Materials, 2021, 20: 1385-1391.
DOI PMID |
| [40] | Wan X, Liu X F, Li Y C, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells[J]. Nature Catalysis, 2019, 2: 259-268. |
| [41] | Padgett E, Yarlagadda V, Holtz M E, et al. Mitigation of PEM fuel cell catalyst degradation with porous carbon supports[J]. Journal of the Electrochemical Society, 2019, 166(4): F198-F207. |
| [42] | Yang C L, Wang L N, Yin P, et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells[J]. Science, 2021, 374(6566): 459-464. |
| [43] | Xiao F, Wang Q, Xu G L, et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells[J]. Nature Catalysis, 2022, 5: 503-512. |
| [44] | Li J K, Sougrati M T, Zitolo A, et al. Identification of durable and non-durable FeNx sites in Fe-N-C materials for proton exchange membrane fuel cells[J]. Nature Catalysis, 2021, 4: 10-19. |
| [45] | Wei J, Xia D S, Wei Y P, et al. Probing the oxygen reduction reaction intermediates and dynamic active site structures of molecular and pyrolyzed Fe-N-C electrocatalysts by in situ Raman spectroscopy[J]. ACS Catalysis, 2022, 12(13): 7811-7820. |
| [46] | Xia D S, Tang X, Dai S, et al. Ultrastable Fe-N-C fuel cell electrocatalysts by eliminating non-coordinating nitrogen and regulating coordination structures at high temperatures[J]. Advanced Materials, 2023, 35(5), doi: 10.1002/adma.202204474. |
| [47] | Zhang X Y, Li L, Cheng K, et al. Directional interface electron transfer from Fe2O3 to biomass-derived carbon originated from F-dopant-induced site-specific growth[J]. Carbon, 2024, 216, doi: 10.1016/j.carbon.2023.118513. |
| [48] | Li J C, Hou P X, Zhao S Y, et al. A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions[J]. Energy & Environmental Science, 2016, 9(10): 3079-3084. |
| [49] | Jeon D, Park J, Shin C, et al. Superaerophobic hydrogels for enhanced electrochemical and photoelectrochemical hydrogen production[J]. Science Advances, 2020, 6(15), doi: 10.1126/sciadv.aaz3944. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||


京公网安备 11010802038735号




